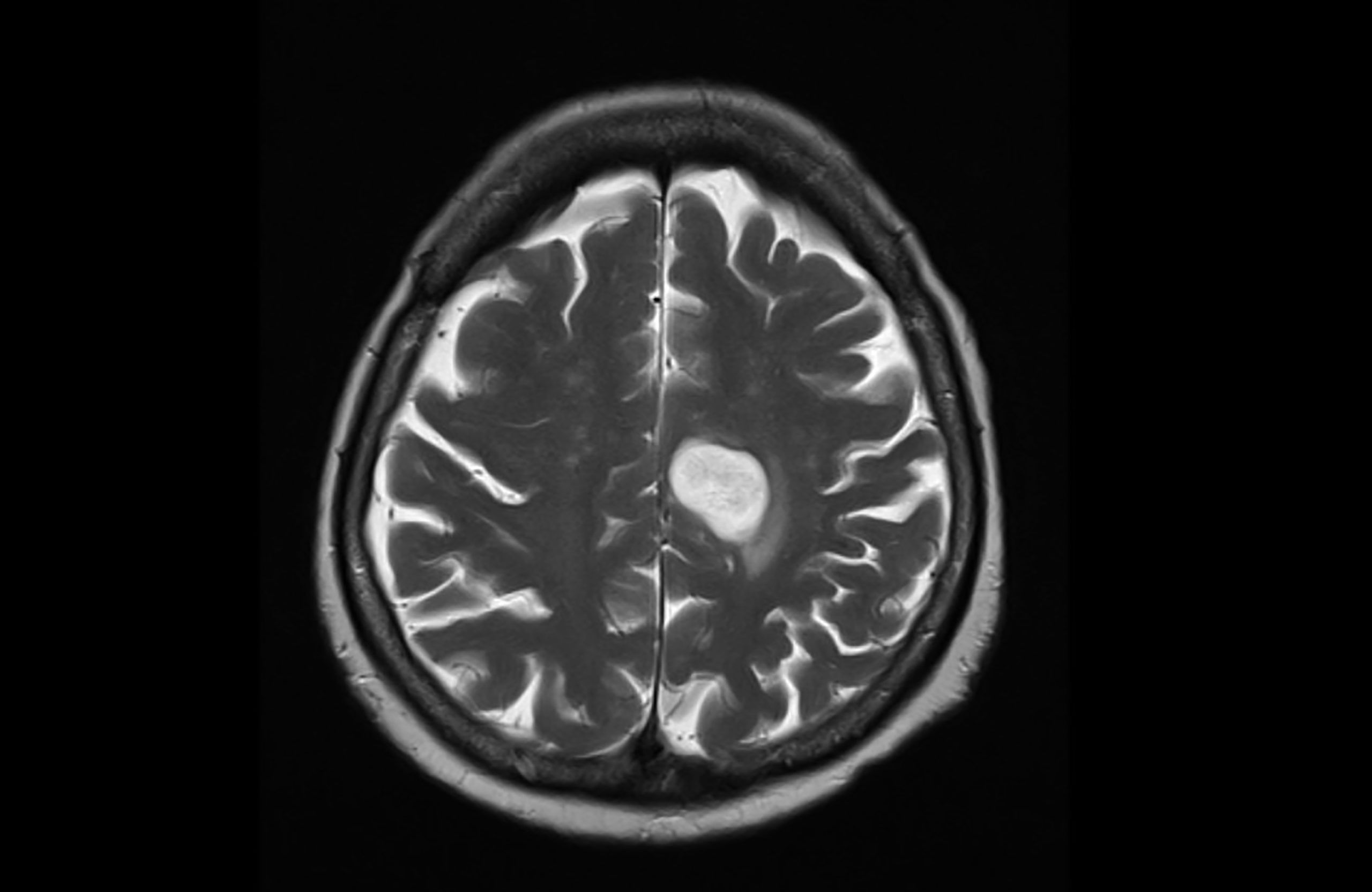
Improving access to evidence-based information for caregivers could contribute to improved outcomes for patients with breast cancer, according to an abstract from the 2022 ASCO Annual Meeting. Terri Kim, of Lunit Inc., Seoul, South Korea, and colleagues asked 330 patients with breast cancer and 53 caregivers to participate in an online survey to better understand various needs throughout the cancer journey. Eight patients and caregivers were asked 25 questions through a remote nurse support model. ...
ASCO 2022 Annual Meeting Conference Coverage
Advertisement
Andrea Sbrana and colleagues presented results of a nationwide, open-label study conducted in 29 Italian centers.
Kathi Mooney, PhD, RN, reported the results of a study looking at the combined intervention at the 2022 ASCO Annual Meeting.
The results were presented at the 2022 American Society of Clinical Oncology (ASCO) Annual Meeting.
Just 38% of patients with breast cancer receive at least 1 palliative care consult following metastatic disease diagnosis.
All stakeholders have a role to play in increasing representation in this patient population.
A study identified self-reported gaps from healthcare providers associated with providing treatment-related support.
Patients with head and neck cancers may derive benefit from receiving oncology nurse navigation.
"Early identification of patient illness may help reduce acute care use and improve quality of care.”
Advertisement